|
|
|
|
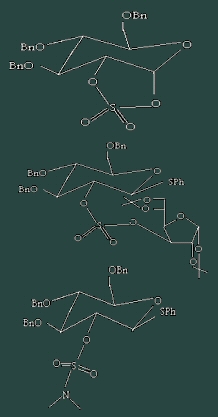
One of the most important reaction in glycochemistry is certainly the glycosylation step during which the link between two saccharides is created. The stereoselectivity of this reaction can be controlled by different protective groups in 2 position. As many polysaccharides possess a sulfate group in this position, the influence of a nearby organosulfate on the orientation of the glycosylation needed to be investigated In this study, diverse derivatives (scheme) were synthesized and tested.
To learn more : web page of the group Supervisor : Dr. Reynald CHEVALLIER Director of the Lab : Pr. Pierre SINAY Address : Ecole Normale Supérieure, 24 rue Lhomond, 75005 PARIS, FRANCE |
|
|
|

Copyright(c) 2005. Webmaster Emma SIERECKI emma_sierecki@yahoo.fr








 Effect of organosulfates on glycosylation
:
Effect of organosulfates on glycosylation
: